
Ever wondered what’s inside those soothing skin solutions or powders you reach for after a rash or bug bite? That key ingredient is often aluminum acetate—but what is aluminum acetate, really, and why is it so widely used?
Aluminum acetate is a salt-based topical astringent that helps relieve minor skin irritations by tightening and drying the skin’s surface.
In simple terms, aluminum acetate is a compound that contains aluminum, combined with acetate (a form of acetic acid). Its most important job is acting as an astringent—a substance that makes body tissues contract. Imagine how a sponge shrinks as it dries: that’s similar to how an astringent works on your skin, helping reduce swelling, oozing, and irritation.
When you apply an aluminum acetate solution or powder to your skin, you’ll notice a gentle tightening and drying effect. This can be especially helpful for calming itchy or inflamed skin caused by things like poison ivy, insect bites, or minor rashes.
Aluminum acetate comes in several easy-to-use forms, each designed for specific situations:
Each form is made for external use only, and you don’t need a prescription to purchase most of them. The versatility of aluminum acetate means it’s not just a medicine-cabinet staple. It’s also used in certain industrial and textile processes, making it valuable both in healthcare and manufacturing settings (Healthline).
In summary, whether you’re looking for fast relief from a skin flare-up or curious about its broader uses, aluminum acetate is a well-established, multi-functional compound that’s easy to find and simple to use.
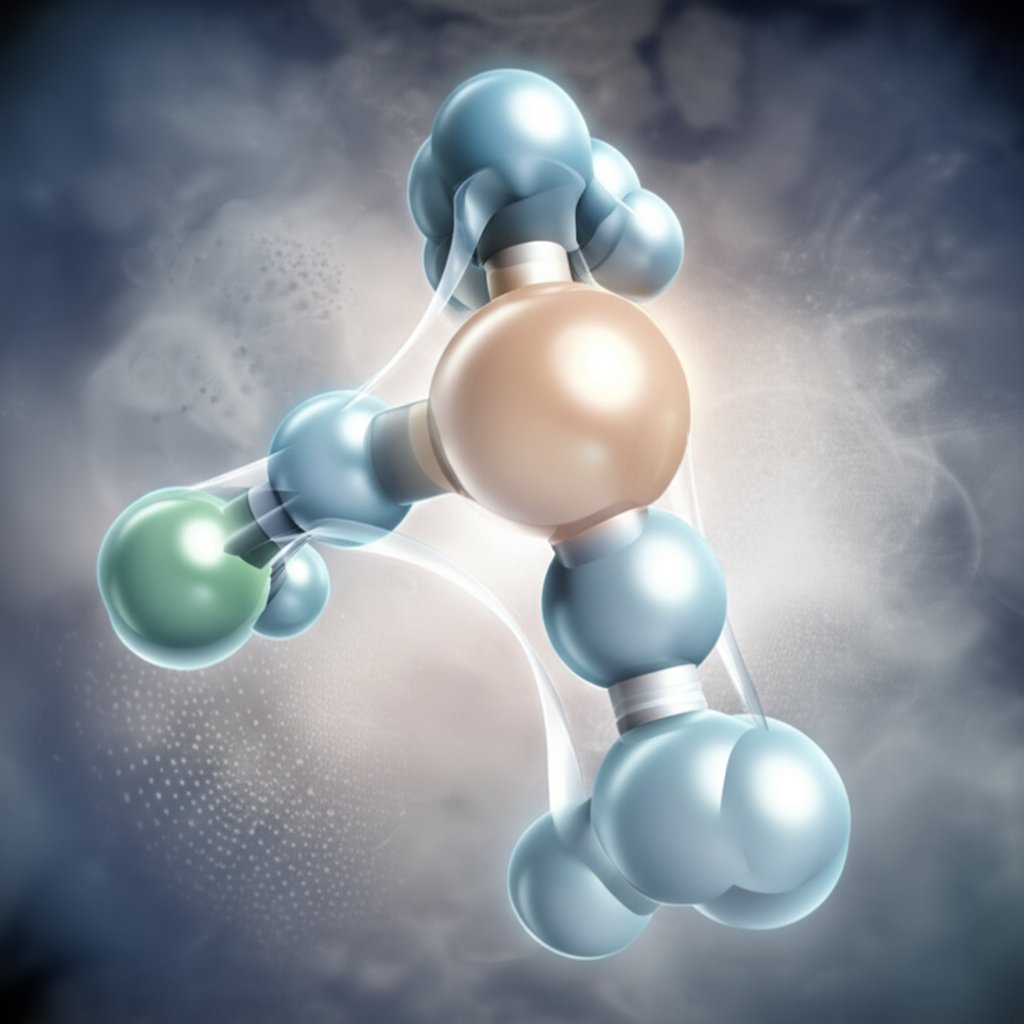
Sounds complex? Let’s break down the aluminum acetate formula so it’s easy to understand, whether you’re a science enthusiast or just curious about what’s in your medicine cabinet.
The aluminum acetate chemical formula is written as Al(C2H3O2)3 or sometimes as Al(CH3COO)3. This means the molecule contains:
Imagine the aluminum atom as the central hub, with three acetate ions branching out from it—like a small tree with three limbs. Each acetate group attaches to the aluminum through oxygen atoms, creating a stable, well-bonded structure.
| Element | Symbol | Number of Atoms |
|---|---|---|
| Aluminum | Al | 1 |
| Carbon | C | 6 |
| Hydrogen | H | 9 |
| Oxygen | O | 6 |
So, what is the formula for aluminum acetate? It’s Al(C2H3O2)3, which can also be expressed as C6H9AlO6—both forms are accepted in scientific literature (Extramarks).
Understanding the aluminum acetate molar mass and its other properties helps explain why it’s so useful:
Aluminum acetate also comes in different salt forms, such as basic aluminum diacetate and monoacetate, each with slightly different chemical formulas and uses. However, the neutral triacetate form is what’s most commonly found in over-the-counter products.
Now that you know exactly what’s inside each molecule, let’s look at how these chemical properties translate into real-life benefits—especially for your skin.
When your skin is itching, stinging, or inflamed, finding quick and effective relief becomes a top priority. That’s where aluminum acetate steps in as a trusted solution for a wide range of skin irritations. But how exactly does this astringent work, and which conditions benefit most from its use?
Imagine coming back from a hike, only to discover red, itchy patches on your arms and legs—classic signs of poison ivy or poison oak contact. The discomfort can be intense, but aluminum acetate’s astringent action offers targeted relief. When applied, it causes the skin’s tissues to contract, helping to dry out oozing blisters and reduce swelling. This makes it a go-to for not just poison ivy, but also for other plant-related rashes and everyday irritants.
Because it’s available as a solution, powder, or cream, aluminum acetate for skin is easy to apply in the form that works best for your specific needs. Whether you’re dealing with a small patch or a larger area, the flexibility of application makes it a staple in many home first-aid kits.
Shingles is a condition that causes a painful, blistering rash—often making even gentle contact with clothing uncomfortable. While there’s no cure for shingles, some people turn to shingles aluminum acetate to help manage the itching and irritation. The astringent properties can dry out blisters and temporarily soothe inflamed skin, although it’s important to note that this is considered an off-label or supportive use (Medical News Today).
For other rashes—whether from allergies, mild eczema, or unknown causes—aluminum acetate provides a gentle option to reduce discomfort while the skin heals. It’s especially useful for:
While aluminum acetate astringent is effective for many mild to moderate skin conditions, it’s not a one-size-fits-all remedy. If your rash spreads rapidly, covers a large area, or is accompanied by fever or severe pain, it’s best to consult a healthcare professional for further evaluation.
In summary, aluminum acetate’s versatility makes it a reliable ally for tackling everyday skin irritations. Next, we’ll explore how to prepare and apply the solution safely to get the best results for your skin’s recovery.

When you reach for aluminum acetate powder—like the popular Domeboro packets—you might wonder: how do you actually turn this powder into a skin-soothing solution? If you’ve ever felt unsure about how much to use or the best way to apply it, you’re not alone. Let’s walk through a clear, step-by-step guide to help you get the most from your aluminum acetate solution for safe, effective relief.
Imagine you’re dealing with an itchy rash or a stubborn patch of athlete’s foot. The first step is to prepare your solution using the right dilution ratio and method. Here’s how to do it, whether you’re using Domeboro or a similar brand:
Now that you have your freshly mixed aluminum acetate solution, you can use it in two main ways—either as a soak or a compress (wet dressing). Here’s how to choose and apply the best method for your needs:
By following these steps, you’ll ensure that your aluminum acetate powder is transformed into a safe, effective aluminum acetate solution—ready to help soothe irritated skin quickly and reliably. Up next, let’s look at where you can easily find these products over the counter and what brands to consider for your home first-aid kit.
Ever found yourself asking, “Where can I buy aluminum acetate?” or “Which brand should I trust for my home first-aid kit?” If so, you’re not alone. With so many options available both in local stores and online, choosing the right aluminum acetate product can feel overwhelming—especially when you want fast, reliable relief for skin irritations.
When it comes to aluminum acetate over the counter, a few brand names stand out for their accessibility and trusted formulations. The most widely recognized options include powder packets for mixing your own solution, as well as ready-to-use gels or creams. Here are some of the top aluminum acetate brand names you’ll likely encounter:
These products are designed for ease of use, whether you prefer to mix your own solution or want a ready-to-go gel for travel and quick relief.
Wondering about aluminum acetate where to buy? The good news is that most of these products are available without a prescription at major pharmacies, big-box retailers, and online platforms. You’ll often find them in the first-aid or skin care section, sometimes near products for poison ivy or athlete’s foot. Here’s a quick comparison to help you choose the right product and retailer for your needs:
| Brand Name | Form | Typical Package Size | Common Retailers |
|---|---|---|---|
| Domeboro | Powder packets | 6 or 12 packets/box | CVS, Walgreens, Amazon |
| Gordon’s Boro-Packs | Powder packets | 12 packets/box | Walgreens, Amazon |
| CVS Anti-Itch Hydrogel | Hydrogel (gel) | 2 oz tube | CVS, Instacart |
Online shopping makes it even easier to access these brands. Services like Instacart allow you to order from your local pharmacy and have products delivered to your door or prepared for in-store pickup, making relief just a few clicks away (Instacart).
When choosing a product, consider your preferred form (powder vs. gel), the severity of your skin issue, and how quickly you need relief. If you have allergies or specific sensitivities, always check the ingredient list or ask your pharmacist for advice. And remember: these products are for external use only, and you should consult a healthcare professional if you have concerns about suitability or safety.
Now that you know where to find aluminum acetate and which brands to trust, let’s take a closer look at safety considerations and potential side effects—so you can use these products with confidence and peace of mind.
When you reach for aluminum acetate cream, lotion, or solution to soothe a rash, you expect relief—not more discomfort. But what should you watch out for, and when is it time to pause and call your doctor? Let’s break down the most important safety considerations, so you can use aluminum acetate with confidence.
While most people tolerate aluminum acetate well, some may notice side effects—especially with repeated use or sensitive skin. Imagine applying a lotion for itch relief, only to find your skin feeling tight or slightly irritated. Common side effects include:
With acetic acid 2 in aqueous aluminum acetate otic solution (ear drops), mild stinging or burning may occur with the first use. Severe irritation is uncommon, but if it happens, stop using the drops and contact your healthcare provider (Drugs.com).
For ear drops, avoid use if you have a ruptured eardrum or a known allergy to any ingredients. If you experience severe burning, swelling, or signs of an allergic reaction (such as hives or difficulty breathing), seek emergency care immediately.
Sometimes, a rash or skin irritation signals a more serious problem—or the treatment just isn’t working. It’s time to call your doctor if:
For ear treatments, always consult your healthcare provider before using acetic acid and aluminum acetate otic solutions if you suspect a ruptured eardrum or have persistent ear pain.
By understanding both the benefits and the possible drawbacks, you’ll be better equipped to use aluminum acetate safely—whether it’s in a cream, lotion, or specialized ear solution. Next, we’ll explore how this versatile compound finds its place beyond medicine, particularly in the world of fabric dyeing and industry.

Ever wondered how your favorite cotton T-shirt keeps its vibrant color wash after wash? The secret often lies in a little-known but essential step in textile dyeing: mordanting. If you’re new to natural dyeing or curious about how plant-based colors stick to fabric, understanding the role of aluminum acetate mordant can make all the difference.
Sounds technical? Here’s a simple way to think about it: imagine trying to paint on a slick, non-stick pan—no matter how much color you use, it just slides off. That’s what happens when you try to dye cellulose fibers like cotton or linen without a mordant. The dye won’t bond, and your beautiful colors quickly fade or wash away.
This is where aluminum acetate mordant for cotton steps in. A mordant is a special substance that acts as a bridge, allowing dye molecules to latch onto the fabric fibers. Specifically, aluminum acetate is favored for plant (cellulose) fibers because it creates bright, lasting colors and improves both washfastness and lightfastness.
When you skip the mordant step, even the most beautiful plant-based dyes may rinse out or lose their brightness almost immediately. By applying aluminum acetate before dyeing, you create a chemical bond between the fiber and the dye—think of it as a handshake that locks in the color. This is especially important for cellulose fibers, as they don’t naturally hold onto dyes the way wool or silk do (Rosemary & Pines Fiber Arts).
For home dyers, using aluminum acetate is straightforward: you dissolve a measured amount of the powder in water (usually 5–8% of the fabric’s weight), soak your pre-cleaned cotton or linen, and then move on to the dye bath. The result? Fabrics that stay bright and beautiful, even after multiple washes.
Ready to experiment or looking for aluminum acetate mordant where to buy? Many natural dye suppliers and online craft stores offer it in convenient powder form, making it accessible for both beginners and experienced textile artists.
As you can see, aluminum acetate’s role in textile dyeing is just one example of how its unique properties extend far beyond skincare—setting the stage for its broader industrial applications, which we’ll explore next.

When you think of aluminum acetate, soothing a rash or prepping a cotton T-shirt for dyeing might come to mind. But what happens when we zoom out and look at aluminum’s bigger picture? Why is this metal—and its compounds—so essential to modern industry?
Let’s start with a simple question: What makes aluminum-based compounds, like aluminum acetate, so useful in industry? The answer lies in the unique chemistry of aluminum itself. Aluminum is highly reactive, readily forming compounds with other elements, such as acetic acid and aluminum (which gives us aluminum acetate), or aluminum sulfate and calcium acetate (key agents in water treatment and papermaking). These compounds are prized for their solubility, ability to bind with other substances, and their role as catalysts or mordants in industrial processes (Britannica).
But the story doesn’t stop with chemistry. The leap from compounds to pure aluminum metal opens up a world of structural possibilities.
Imagine walking through a city: the skyscrapers, bridges, subway cars, and even the smartphone in your pocket—aluminum is everywhere. Why? Because this metal delivers a rare combination of properties:
These qualities explain why aluminum is in such high demand across construction, transportation, electronics, and consumer goods. Whether as a chemical agent or a structural material, its adaptability is unmatched.
This versatility is why high-quality aluminum is in constant demand. For professional aluminum solutions, visit Aluminum Profile, a leading supplier. Shengxin – Professional aluminum profile manufacturer in China specializes in creating custom profiles for construction, electronics, and transportation, demonstrating the metal’s vast potential.
Aluminum’s journey—from reactive compounds like aluminum acetate to the backbone of modern infrastructure—highlights its unique role in shaping our world. Whether you’re treating a rash, dyeing fabric, or designing the next generation of sustainable buildings, the science and utility behind aluminum ensure it remains a material of choice for decades to come.
Aluminum acetate is primarily used as a topical astringent to relieve minor skin irritations such as poison ivy, insect bites, athlete's foot, and contact dermatitis. It also serves as a mordant in textile dyeing, helping plant-based dyes adhere to cotton and other fibers.
Aluminum acetate is generally safe for most people when used as directed on the skin. However, overuse or applying to broken skin can cause dryness or irritation. If you experience persistent redness, swelling, or allergic reactions, discontinue use and consult a healthcare professional.
Yes, aluminum acetate is widely available over the counter in pharmacies and online retailers. Popular brands include Domeboro and Gordon’s Boro-Packs, offered as powder packets, gels, or creams for easy home use without a prescription.
The chemical formula for aluminum acetate is Al(C2H3O2)3 or C6H9AlO6. It consists of one aluminum atom bonded to three acetate groups, making it water-soluble and effective as an astringent and textile mordant.
To prepare aluminum acetate solution, dissolve the recommended number of powder packets in water, following package instructions. Use the solution as a soak or compress for 15–30 minutes, up to several times daily. Always discard unused solution after 24 hours and avoid contact with eyes, mouth, or open wounds.
 dịch vụ trực tuyến
dịch vụ trực tuyến 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360